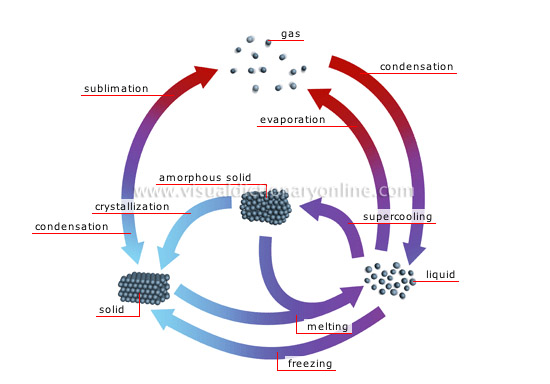
states of matter
Matter exists in three fundamental states (solid, liquid and gaseous), which depend on the temperature and pressure to which the matter is subjected.
amorphous solid 
Body that resembles a congealed liquid whose atoms are not ordered.
gas 
Malleable and expandable matter whose only definable property is mass; its atoms are fully mobile with respect to each other.
crystallization 
Change of a substance from an amorphous state to a crystallized state; it results from cooling, which causes the atoms to become ordered.
condensation 
Change of a substance from a gaseous state to a liquid state; it results from cooling.
evaporation 
Change of a substance from a liquid state to a gaseous state; it results from heating.
melting 
Change of a substance from a solid state to a liquid state; it results from heating.
freezing 
Change of a substance from a liquid state to a solid state; it results from cooling.
sublimation 
Change of a substance from a solid state directly to a gaseous state without passing through the liquid state; it results from heating.
liquid 
Matter having a definite mass and volume but no shape; its atoms are relatively mobile in relation to each other.
condensation 
Change of a substance from a gaseous state to a liquid state; it results from cooling.
solid 
Rigid body possessing mass, volume and a definite form; its atoms are linked to each other and are almost completely at rest.
supercooling 
The process of cooling a liquid below the point at which it normally freezes (solidifies); its atoms become unstable.