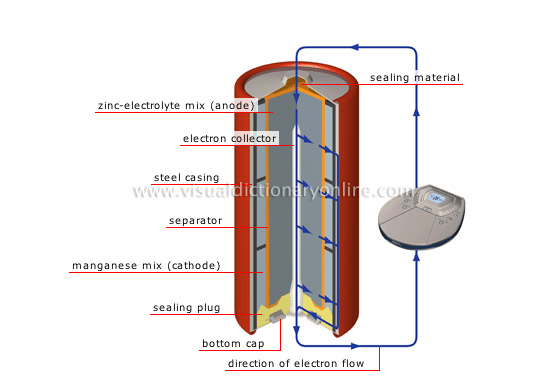
alkaline manganese-zinc cell
High-performance battery that produces 1.5 V and has a longer life span than the carbon-zinc cell; it is used in devices such as flashlights, portable CD players and camera flash units.
direction of electron flow 
When a chemical reaction occurs, the electrons move from the negative terminal toward the positive terminal, thus creating an electric current.
zinc-electrolyte mix (anode) 
Substance that is made up of zinc and electrolyte (potassium hydroxide); it constitutes the positive electrode (anode).
electron collector 
Zinc rod that is connected to the bottom cap; it collects the electrons from the anode that are attracted to the cathode.
steel casing 
Covering that protects the battery.
separator 
Porous paper combined with a chemical paste (potassium hydroxide) that separates the two electrodes; this allows electrons to pass, thus conducting electricity.
manganese mix (cathode) 
Substance made up of manganese dioxide and carbon; it constitutes the negative electrode (cathode).
sealing plug 
Material that seals the battery.
bottom cap 
Lower metal cover; the negative terminal is located at its center.
sealing material 
Material (nylon) that seals the battery.